Changes in Pain Sensitivity in Treatment for Breast Cancer: A 12-Month Follow-Up Case Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
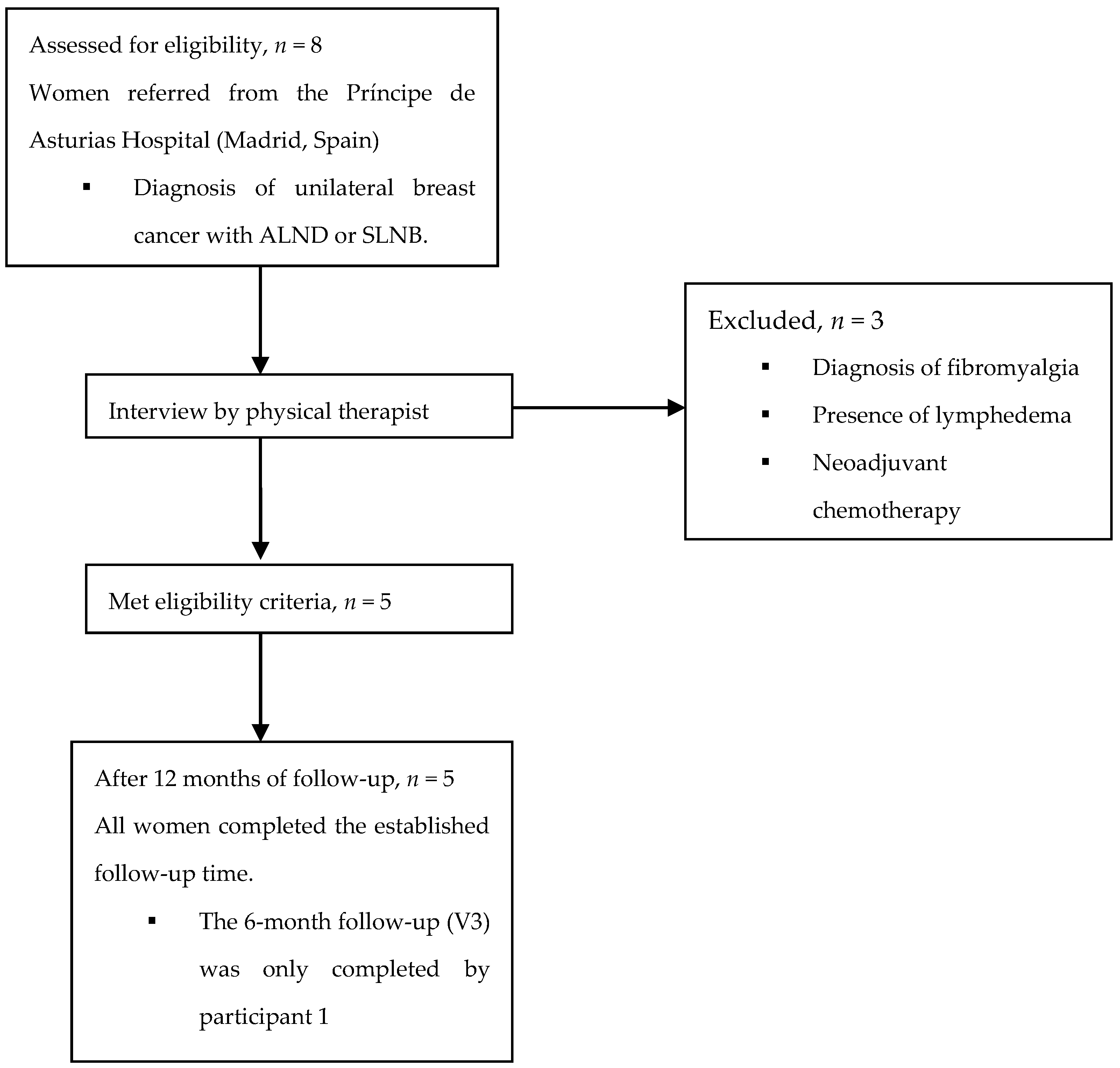
2.2. Participants
2.3. Assessment Procedure
2.4. Outcome Measures
2.4.1. Direct Measurements of Pain Sensitization
Mechanical Detection Threshold (MDT)
Vibration Detection Threshold (VDT)
Warm and Cold Detection and Pain
Temporal Summation (TS)
Pressure Pain Threshold (PPT)
Suprathreshold Stimulus
2.4.2. Indirect Measurement of Pain Sensitization
2.4.3. Pain
2.4.4. Data Analysis
3. Results
4. Discussion
4.1. Direct Measurements of Sensitization
4.2. Indirect Measurement of Sensitization
4.3. Pain
4.4. Symptomatology and Treatments
4.5. Strengths and Limitations
4.6. Future Research Lines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Konieczny, M.; Cipora, E.; Sygit, K.; Fal, A. Quality of life of women with breast cancer and socio-demographic factors. Asian Pac. J. Cancer Prev. 2020, 21, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Cohen, J.C.; Devasenapathy, N.; Hong, B.Y.; Kheyson, S.; Lu, D.; Oparin, Y.; Kennedy, S.A.; Romerosa, B.; Arora, N.; et al. Prevalence and intensity of persistent post-surgical pain following breast cancer surgery: A systematic review and meta-analysis of observational studies. Br. J. Anaesth. 2020, 125, 346–357. [Google Scholar] [CrossRef]
- Leysen, L.; Beckwée, D.; Nijs, J.; Pas, R.; Bilterys, T.; Vermeir, S.; Adriaenssens, N. Risk factors of pain in breast cancer survivors: A systematic review and meta-analysis. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2017, 25, 3607–3643. [Google Scholar] [CrossRef] [PubMed]
- van Helmond, N.; Timmerman, H.; van Dasselaar, N.T.; van de Pol, C.C.; Olesen, S.S.; Drewes, A.M.; Vissers, K.; Wilder-Smith, O.H.; Steegers, M.A. High body mass index is a potential risk factor for persistent postoperative pain after breast cancer treatment. Pain Phys. 2017, 20, E661–E671. [Google Scholar]
- Wang, K.; Yee, C.; Tam, S.; Drost, L.; Chan, S.; Zaki, P.; Rico, V.; Ariello, K.; Dasios, M.; Lam, H.; et al. Prevalence of pain in patients with breast cancer post-treatment: A systematic review. Breast 2018, 42, 113–127. [Google Scholar] [CrossRef]
- Juhl, A.A.; Christiansen, P.; Damsgaard, T.E. Persistent pain after breast cancer treatment: A questionnaire-based study on the prevalence, associated treatment variables, and pain type. J. Breast Cancer 2016, 19, 447–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besic, N.; Smrekar, J.; Strazisar, B. Acute pain and side effects after tramadol in breast cancer patients: Results of a prospective double-blind randomized study. Sci. Rep. 2020, 10, 18766. [Google Scholar] [CrossRef] [PubMed]
- Chiang, D.L.C.; Rice, D.A.; Helsby, N.A.; Somogyi, A.A.; Kluger, M.T. The prevalence, impact, and risk factors for persistent pain after breast cancer surgery in a New Zealand population. Pain Med. 2019, 20, 1803–1814. [Google Scholar] [CrossRef]
- Schreier, A.M.; Johnson, L.A.; Vohra, N.A.; Muzaffar, M.; Kyle, B. Post-treatment symptoms of pain, anxiety, sleep disturbance, and fatigue in breast cancer survivors. Pain Manag. Nurs. Off. J. Am. Soc. Pain Manag. Nurs. 2019, 20, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Torres Lacomba, M.; Mayoral Del Moral, O.; Coperias Zazo, J.L.; Yuste Sánchez, M.J.; Ferrandez, J.C.; Zapico Goñi, A. Axillary web syndrome after axillary dissection in breast cancer: A prospective study. Breast Cancer Res. Treat. 2009, 117, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Divella, M.; Vetrugno, L.; Bertozzi, S.; Seriau, L.; Cedolini, C.; Bove, T. Patient-reported pain and other symptoms among breast cancer survivors: Prevalence and risk factors. Tumori 2020, 106, 480–490. [Google Scholar] [CrossRef]
- Schreiber, K.L.; Zinboonyahgoon, N.; Xu, X.; Spivey, T.; King, T.; Dominici, L.; Partridge, A.; Golshan, M.; Strichartz, G.; Edwards, R.R. Preoperative psychosocial and psychophysical phenotypes as predictors of acute pain outcomes after breast surgery. J. Pain 2019, 20, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Leysen, L.; Adriaenssens, N.; Nijs, J.; Pas, R.; Bilterys, T.; Vermeir, S.; Lahousse, A.; Beckwée, D. Chronic pain in breast cancer survivors: Nociceptive, neuropathic, or central sensitization pain? Pain Pract. Off. J. World Inst. Pain 2019, 19, 183–195. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa-Díaz, I.; Torres-Lacomba, M.; Acosta-Ramírez, P.; Orive, I.G.; Nee, R.J.; de la Villa-Polo, P.; Andrés-Esteban, E.M.; Sánchez-Sánchez, B. Protective myoelectric activity at performing upper limb neurodynamic test 1 in breast cancer survivors. A cross-sectional observational study. Musculoskelet. Sci. Pract. 2018, 36, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, E.; Chee, E.; Hush, J.; Moloney, N. The prevalence of neuropathic pain is high after treatment for breast cancer: A systematic review. Pain 2017, 158, 2082–2091. [Google Scholar] [CrossRef] [PubMed]
- Koulouris, A.E.; Edwards, R.R.; Dorado, K.; Schreiber, K.L.; Lazaridou, A.; Rajan, S.; White, J.; Garcia, J.; Gibbons, C.; Freeman, R. Reliability and validity of the boston bedside quantitative sensory testing battery for neuropathic pain. Pain Med. 2020, 21, 2336–2347. [Google Scholar] [CrossRef] [PubMed]
- Torres Lacomba, M.; Mayoral del Moral, O.; Coperias Zazo, J.L.; Gerwin, R.D.; Goñí, A.Z. Incidence of myofascial pain syndrome in breast cancer surgery: A prospective study. Clin. J. Pain 2010, 26, 320–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manfuku, M.; Nishigami, T.; Mibu, A.; Tanaka, K.; Kitagaki, K.; Sumiyoshi, K. Comparison of central sensitization-related symptoms and health-related quality of life between breast cancer survivors with and without chronic pain and healthy controls. Breast Cancer 2019, 26, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Feeney, L.R.; Tormey, S.M.; Harmon, D.C. Breast cancer and chronic pain: A mixed methods review. Ir. J. Med. Sci. 2018, 187, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Gierthmühlen, J.; Schneider, U.; Seemann, M.; Freitag-Wolf, S.; Maihöfner, C.; Enax-Krumova, E.K.; Azad, S.C.; Üçeyler, N.; Birklein, F.; Maier, C.; et al. Can self-reported pain characteristics and bedside test be used for the assessment of pain mechanisms? An analysis of results of neuropathic pain questionnaires and quantitative sensory testing. Pain 2019, 160, 2093–2104. [Google Scholar] [CrossRef]
- Treede, R.D. The role of quantitative sensory testing in the prediction of chronic pain. Pain 2019, 160 (Suppl. 1), S66–S69. [Google Scholar] [CrossRef] [PubMed]
- Martland, M.E.; Rashidi, A.S.; Bennett, M.I.; Fallon, M.; Jones, C.; Rolke, R.; Mulvey, M.R. The use of quantitative sensory testing in cancer pain assessment: A systematic review. Eur. J. Pain 2020, 24, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Rolke, R.; Baron, R.; Maier, C.; Tölle, T.R.; Treede, D.R.; Beyer, A.; Binder, A.; Birbaumer, N.; Birklein, F.; Bötefür, I.C.; et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 2006, 123, 231–243. [Google Scholar] [CrossRef]
- Andersen, K.G.; Kehlet, H.; Aasvang, E.K. Test-retest agreement and reliability of quantitative sensory testing 1 year after breast cancer surgery. Clin. J. Pain 2015, 31, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.G.; Duriaud, H.M.; Kehlet, H.; Aasvang, E.K. The relationship between sensory loss and persistent pain 1 year after breast cancer surgery. J. Pain 2017, 18, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, L.; Vollert, J.; Rice, A.S.C.; Kalso, E.; Harno, H. Sensory profiles in women with neuropathic pain after breast cancer surgery. Breast Cancer Res. Treat. 2020, 182, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, V.; Akin-Akinyosoye, K.; Zhang, W.; McWilliams, D.F.; Hendrick, P.; Walsh, D.A. Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: A systematic review and meta-analysis. Pain 2019, 160, 1920–1932. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.G.; Duriaud, H.M.; Aasvang, E.K.; Kehlet, H. Association between sensory dysfunction and pain 1 week after breast cancer surgery: A psychophysical study. Acta Anaesthesiol. Scand. 2016, 60, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Kanzawa-Lee, G.A.; Harte, S.E.; Bridges, C.M.; Brummett, C.; Clauw, D.J.; Williams, D.A.; Knoerl, R.; Lavoie Smith, E.M. Pressure pain phenotypes in women before breast cancer treatment. Oncol. Nurs. Forum 2018, 45, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Hershman, D.L.; Weimer, L.H.; Wang, A.; Kranwinkel, G.; Brafman, L.; Fuentes, D.; Awad, D.; Crew, K.D. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res. Treat. 2011, 125, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Zhi, W.I.; Baser, R.E.; Kwon, A.; Chen, C.; Li, S.Q.; Piulson, L.; Seluzicki, C.; Panageas, K.S.; Harte, S.E.; Mao, J.J.; et al. Characterization of chemotherapy-induced peripheral neuropathy using patient-reported outcomes and quantitative sensory testing. Breast Cancer Res. Treat. 2021, 186, 761–768. [Google Scholar] [CrossRef]
- Krøigård, T.; Schrøder, H.D.; Qvortrup, C.; Eckhoff, L.; Pfeiffer, P.; Gaist, D.; Sindrup, S.H. Characterization and diagnostic evaluation of chronic polyneuropathies induced by oxaliplatin and docetaxel comparing skin biopsy to quantitative sensory testing and nerve conduction studies. Eur. J. Neurol. 2014, 21, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: Explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Craggs, J.G.; Bialosky, J.E.; Bishop, M.D.; George, S.Z.; Staud, R.; Robinson, M.E. Temporal summation of second pain: Variability in responses to a fixed protocol. Eur. J. Pain 2013, 17, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Arroyo-Fernandez, R.; Bravo-Esteban, E.; Domenech-Garcia, V.; Ferri-Morales, A. Pressure-induced referred pain as a biomarker of pain sensitivity in fibromyalgia. Pain Phys. 2020, 23, E353–E362. [Google Scholar]
- Cuesta-Vargas, A.I.; Roldan-Jimenez, C.; Neblett, R.; Gatchel, R.J. Cross-cultural adaptation and validity of the Spanish central sensitization inventory. SpringerPlus 2016, 5, 1837. [Google Scholar] [CrossRef] [Green Version]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. 11), S240–S252. [Google Scholar]
- López-de-Uralde-Villanueva, I.; Gil-Martínez, A.; Candelas-Fernández, P.; de Andrés-Ares, J.; Beltrán-Alacreu, H.; La Touche, R. Validity and reliability of the Spanish-language version of the self-administered Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) pain scale. Neurologia 2018, 33, 505–514. [Google Scholar] [CrossRef]
- Jensen, M.P.; Turner, J.A.; Romano, J.M. What is the maximum number of levels needed in pain intensity measurement? Pain 1994, 58, 387–392. [Google Scholar] [CrossRef]
- Caraceni, A.; Cherny, N.; Fainsinger, R.; Kaasa, S.; Poulain, P.; Radbruch, L.; De Conno, F. Pain measurement tools and methods in clinical research in palliative care: Recommendations of an Expert Working Group of the European Association of Palliative Care. J. Pain Symptom Manage. 2002, 23, 239–255. [Google Scholar] [CrossRef]
- Dougherty, P.M.; Cata, J.P.; Cordella, J.V.; Burton, A.; Weng, H.R. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain 2004, 109, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.L.; Mulvey, M.R. Cancer-related neuropathic pain. Cancers 2019, 11, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puonti, H.K.; Broth, T.A.; Soinila, S.O.; Hallikainen, H.K.; Jääskeläinen, S.K. How to assess sensory recovery after breast reconstruction surgery? Clin. Breast Cancer 2017, 17, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.E.; Bialosky, J.E.; Bishop, M.D.; Price, D.D.; George, S.Z. Supra-threshold scaling, temporal summation, and after-sensation: Relationships to each other and anxiety/fear. J. Pain Res. 2010, 3, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, C.; Cornelius, V.; Love, S.; Graham, J.; Richards, M.; Ramirez, A. Depression and anxiety in women with early breast cancer: Five year observational cohort study. BMJ 2005, 330, 702. [Google Scholar] [CrossRef] [Green Version]
- Gottrup, H.; Andersen, J.; Arendt-Nielsen, L.; Jensen, T.S. Psychophysical examination in patients with post-mastectomy pain. Pain 2000, 87, 275–284. [Google Scholar] [CrossRef]
- Staud, R.; Craggs, J.G.; Robinson, M.E.; Perlstein, W.M.; Price, D.D. Brain activity related to temporal summation of C-fiber evoked pain. Pain 2007, 129, 130–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Groef, A.; Meeus, M.; De Vrieze, T.; Vos, L.; Van Kampen, M.; Geraerts, I.; Devoogdt, N. Unraveling self-reported signs of central sensitization in breast cancer survivors with upper limb pain: Prevalence rate and contributing factors. Pain Phys. 2018, 21, E247–E256. [Google Scholar] [CrossRef]
- Schug, S.A.; Lavand’homme, P.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D. The IASP classification of chronic pain for ICD-11: Chronic postsurgical or posttraumatic pain. Pain 2019, 160, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Fontes, F.; Sonin, T.; Dias, T.; Fragoso, M.; Castro-Lopes, J.; Lunet, N. Neuropathic pain after breast cancer treatment: Characterization and risk factors. J. Pain Symptom Manage. 2017, 54, 877–888. [Google Scholar] [CrossRef] [Green Version]
- Roldán-Jiménez, C.; Pérez-Cruzado, D.; Neblett, R.; Gatchel, R.; Cuesta-Vargas, A. Central sensitization in chronic musculoskeletal pain disorders in different populations: A cross-sectional study. Pain Med. 2020, 21, 2958–2963. [Google Scholar] [CrossRef] [PubMed]
- van Helmond, N.; Aarts, H.M.; Timmerman, H.; Olesen, S.S.; Drewes, A.M.; Wilder-Smith, O.H.; Steegers, M.A.; Vissers, K.C. Is preoperative quantitative sensory testing related to persistent postsurgical pain? A systematic literature review. Anesth. Analg. 2020, 131, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.J.; Robinson, P.J.; Nazeem, F.; Panjari, M.; Fradkin, P.; Schwarz, M.; Davis, S.R. Persistent breast pain 5 years after treatment of invasive breast cancer is largely unexplained by factors associated with treatment. J. Cancer Surviv. 2014, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guyatt, G.H.; Kennedy, S.A.; Romerosa, B.; Kwon, H.Y.; Kaushal, A.; Chang, Y.; Craigie, S.; de Almeida, C.P.B.; Couban, R.J.; et al. Predictors of persistent pain after breast cancer surgery: A systematic review and meta-analysis of observational studies. CMAJ 2016, 188, E352–E361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, R.; Jing, J.; Zhang, X.; Li, M.; Gao, J. Adherence to post-surgery follow-up assessment and its association with sociodemographic and disease characteristics in patients with breast cancer in Central China. BMC Cancer 2020, 20, 1098. [Google Scholar] [CrossRef] [PubMed]


| Participants | |||||
|---|---|---|---|---|---|
| Characteristics | 1 | 2 | 3 | 4 | 5 |
| Age (years) | 51 | 43 | 36 | 49 | 39 |
| BMI (kg/m2) | 32.05 | 30.48 | 21.11 | 18.20 | 18.34 |
| Menopause | Yes | No | No | No | No |
| Affected side | Left | Left | Right | Right | Left |
| Affected upper limbs | Yes | No | No | Yes | No |
| Previous shoulder pathology | Impingement | No | No | Painful shoulder | No |
| Limited motion of the shoulder | Yes | No | No | Yes | No |
| Number of people in the household | 1 | 4 | 4 | 2 | 3 |
| Children | 0 | 2 | 2 | 0 | 0 |
| Adults | 1 | 2 | 2 | 2 | 3 |
| Educational level | University | Non-completed secondary education | University | High school | High school |
| Yearly income (EUR) | <12,000 | Did not answer | >48,000 | 12,000–24,000 | 24,000–36,000 |
| Surgical procedure | |||||
| Mastectomy plus immediate reconstruction Lumpectomy | Yes No | Yes No | Yes No | No Yes | Yes No |
| Axillary dissection procedure | |||||
| ALND SLNB | No Yes | No Yes | No Yes | No Yes | Yes No |
| Postoperative therapy | |||||
| Chemotherapy | Yes | No | No | No | Yes |
| Radiotherapy | Yes | No | No | No | Yes |
| Hormone therapy | Letrozole | Letrozole | No | Letrozole | Tamoxifen |
| Participants | ||||||
|---|---|---|---|---|---|---|
| Measurement | V | 1 | 2 | 3 | 4 | 5 |
| PAIN | V0 | Yes | No | No | No | No |
| V1 | Yes | Yes | Yes | Yes | Yes | |
| V2 | Yes | No | Yes | No | No | |
| V3 | Yes | * | * | * | * | |
| V4 | Yes | No | No | No | Yes | |
| V5 | Yes | No | No | No | Yes | |
| NRS (%) | V0 | 70 | NP | NP | NP | NP |
| V1-V0 | 0 | 10 (10) | 0 | 80 (80) | 20 (20) | |
| V2-V0 | 0 | 0 | 0 | 0 | 0 | |
| V3-V0 | 0 | * | * | * | * | |
| V4-V0 | −30 (−30) | 0 | 0 | 0 | 60 (60) | |
| V5-V0 | −10 (−10) | 0 | 0 | 0 | 70 (70) | |
| LOCATION | V0 | 1 | NP | NP | NP | NP |
| V1 | 3 | 2, 5 | 3 | 3 | 3 | |
| V2 | 3 | NP | 3 | NP | NP | |
| V3 | 3 | * | * | * | * | |
| V4 | 0 | NP | NP | NP | 4, 6 | |
| V5 | 3 | NP | NP | NP | 7 | |
| Participants | |||||||
|---|---|---|---|---|---|---|---|
| Measurement | V | 1 | 2 | 3 | 4 | 5 | |
| TS | Wind-up ratio affected side | V0 | 3.38 | 1.32 | 2.5 | 6 | 1.41 |
| V1-V0 | −2.3 (−68.05) | 0.31 (23.48) | 0.3 (12) | −4.56 (−76) | −0.10 (−7.6) | ||
| V2-V0 | −2.31 (−68.34) | 0.01 (0.76) | 1.17 (46.8) | −4.65 (−77.5) | −0.11 (−7.8) | ||
| V3-V0 | −1.72 (−50.89) | * | * | * | * | ||
| V4-V0 | −2.26 (−66.86) | −0.01 (−0.76) | 0.93 (37.2) | −4.45 (−74.17) | −0.08 (−5.67) | ||
| V5-V0 | −2.38 (−70.41) | 0.12 (9.09) | −1.21 (−48.4) | −4.75 (−79.17) | −0.39 (−27.66) | ||
| Wind-up ratio non-affected side | V0 | 4 | 1.3 | 1.83 | 3 | 1.64 | |
| V1-V0 | −2.89 (−72.25) | 0.20 (15.38) | 0.50 (27.32) | −1.32 (−44) | −0.23 (−15.02) | ||
| V2-V0 | −2.86 (−71.5) | 0.03 (2.31) | 1.06 (57.92) | −1.92 (−64) | −0.28 (−17.07) | ||
| V3-V0 | −2.4 (−60) | * | * | * | * | ||
| V4-V0 | −2.9 (−72.5) | 0.24 (18.46) | 1.17 (63.93) | −1.38 (−46) | −0.41 (−25) | ||
| V5-V0 | −2.95 (−73.75) | 0.01 (0.77) | −0.59 (−32.24) | −1.58 (−52.67) | −0.57 (−34.76) | ||
| STPS | V0 | 0 | 0 | 1 | 3 | 5 | |
| V1-V0 | 6 (100) | 0 (0) | 2 (100) | 4 (100) | * | ||
| V2-V0 | 0 (0) | 0 (0) | 0 (100) | 4 (100) | 0 (100) | ||
| V3-V0 | 0 (0) | * | * | * | * | ||
| V4-V0 | 1 (100) | 0 (0) | 3 (100) | 4 (100) | 0 (100) | ||
| V5-V0 | 0 (0) | 0 (0) | 0 (100) | 5 (100) | 0 (100) | ||
| VDT | Acromion affected side (s) | V0 | 10 | 15 | 12 | 12 | 20.59 |
| V1-V0 | −6 (42.86) | 9 (47.37) | 5 (20) | 6 (35.29) | 3 (16.22) | ||
| V2-V0 | 3 (21.43) | 0 | 10 (40) | −4 (−23.53) | 3.39 (16.46) | ||
| V3-V0 | −1 (−7.14) | * | * | * | * | ||
| V4-V0 | −5 (−35.71) | 3 (15.79) | 15 (60) | 7 (41.18) | 1 (4.82) | ||
| V5-V0 | 4 (28.57) | 5 (26.32) | 8 (32) | 3 (17.65) | −3 (−14.57) | ||
| Epycondile affected side (s) | V0 | 7 | 22 | 22 | 7 | 32 | |
| V1-V0 | −1 (−11.11) | −9 (−37.5) | −4 (−12.12) | 6 (46.15) | −10.2 (−37.8) | ||
| V2-V0 | 2 (22.22) | −16 (−66.67) | −7 (−21.21) | −3 (−23.08) | −12.8 (−40) | ||
| V3-V0 | 0 | * | * | * | * | ||
| V4-V0 | −1 (−11.11) | 2 (8.32) | 11 (33.33) | 3 (23.08) | −20 (−62.5) | ||
| V5-V0 | −4 (−44.44) | −14 (−58.33) | −1 (−3.03) | 3 (23.08) | −24 (−75) | ||
| Styloide affected side (s) | V0 | 22 | 23 | 36 | 16 | 20 | |
| V1-V0 | 0 | 2 (5.41) | −11 (−20) | 12 (42.86) | 0 | ||
| V2-V0 | −3 (−13.64) | −1 (−2.7) | −5 (−9.09) | −9 (−32.14) | 4 (16.67) | ||
| V3-V0 | −3 (−13.64) | * | * | * | * | ||
| V4-V0 | −18 (−81.82) | 14 (37.84) | 19 (34.55) | 7 (25) | 3 (12.5) | ||
| V5-V0 | −6 (−27.27) | −8 (−21.62) | −6 (−10.91) | 5 (17.86) | 2 (8.33) | ||
| Acromion non-affected side (s) | V0 | 6 | 17 | 14 | 7 | 17 | |
| V1-V0 | −2 (−13.33) | 1 (5.56) | 4 (15.38) | 8 (53.33) | −4.7 (−27) | ||
| V2-V0 | 7 (46.67) | −7 (−38.89) | 7 (26.92) | −4 (−26.67) | −6.8 (−40) | ||
| V3-V0 | 1 (6.67) | * | * | * | * | ||
| V4-V0 | −1 (−6.67) | −3 (−16.67) | 12 (46.15) | 6 (40) | −6 (−35.29) | ||
| V5-V0 | 9 (60) | −10 | 4 | 5 | −8 (−47.06) | ||
| Epicondyle non-affected side (s) | V0 | 6 | 17 | 14 | 7 | 17 | |
| V1-V0 | 4 (38.36) | 2 (14.29) | −4 (−13.79) | 2 (15.5) | −10.4 (−61.17) | ||
| V2-V0 | 2 (18.18) | −4 (−28.57) | −4 (−13.79) | −8 (−50) | −14.1 (−55.53) | ||
| V3-V0 | 0 | * | * | * | * | ||
| V4-V0 | −1 (−9.09) | 2 (14.29) | 10 (34.48) | 6 (37.5) | −15.39(−6.61) | ||
| V5-V0 | −3 (−27.27) | −6 (−42.86) | 5 (17.24) | 5 (31.25) | −16.39 (−64.65) | ||
| Styloide non-affected side (s) | V0 | 14 | 23 | 32 | 15 | 12.4 | |
| V1-V0 | 9 (88.46) | 1 (2.94) | −4 (−10.53) | 10 (7.03) | −0.44 (−3.54) | ||
| V2-V0 | 12 (100) | 1 (2.94) | 0 | −6 (−22.22) | −0.91 (−4.79) | ||
| V3-V0 | 5 (73.08) | * | * | * | * | ||
| V4-V0 | −8 (23.08) | 11 (32.35) | 6 (15.79) | 12 (44.44) | 5.6 (29.47) | ||
| V5-V0 | 5 (73.08) | −11 (−32.35) | −2 (−5.26) | 10 (37.03) | 6.6 (3.74) | ||
| Participants | |||||||
|---|---|---|---|---|---|---|---|
| Measurement | V | 1 | 2 | 3 | 4 | 5 | |
| MDT | Affected side increasing (g) | V0 | 0.16 | 0.6 | 1 | 2 | 0.4 |
| V1-V0 | 0 | 0.4 (40) | 0 | 0 | 0 | ||
| V2-V0 | 0.24 (60) | −0.44 (−44) | −0.84 (−84) | 0 | −0.36 (−90) | ||
| V3-V0 | 0 | * | * | * | * | ||
| V4-V0 | −0.12 (−30) | 0 | −0.6 (−60) | −1.4 (−70) | 0 | ||
| V5-V0 | 0.24 (60) | 0.4 (40) | −0.6 (−60) | −1.4 (−70) | 0 | ||
| Affected side decreasing (g) | V0 | 0.16 | 0.16 | 0.6 | 0.6 | 0.16 | |
| V1-V0 | 15.84 (99) | 0.24 (40) | −0.44 (−73.33) | 1.4 (70) | 0.24 (40) | ||
| V2-V0 | −0.09 (−0.56) | 0.24 (40) | −0.44 (−73.33) | 0.4 (20) | −0.152 (−95) | ||
| V3-V0 | −0.09 (−0.56) | * | * | * | * | ||
| V4-V0 | −0.14 (−0.88) | 0.44 (73.33) | −0.44 (−73.33) | −0.2 (−10) | 0 | ||
| V5-V0 | −0.09 (−0.56) | 0.24 (40) | −0.44 (−73.33) | −0.2 (−10) | 0 | ||
| Non-affected side increasing (g) | V0 | 0.16 | 0.4 | 1 | 1.4 | 0.16 | |
| V1-V0 | 0.24 (60) | 0 | −0.6 (−60) | −1.24 (−88.57) | 0.12 (30) | ||
| V2-V0 | −0.12 (−30) | 0 | −0.84 (−54) | −1 (−71.43) | 0.24 (60) | ||
| V3-V0 | 0.24 (60) | * | * | * | * | ||
| V4-V0 | −0.12 (−30) | 0.2 (33.33) | −0.6 (−60) | −0.8 (−57.14) | 0 | ||
| V5-V0 | 0.24 (60) | 0.2 (33.33) | −0.6 (−60) | −1 (−71.43) | 0 | ||
| Non-affected side decreasing (g) | V0 | 0.07 | 0.16 | 0.6 | 0.6 | 0.16 | |
| V1-V0 | 0.09 (22.5) | −0.09 (−22.5) | −0.53 (−88.33) | 0.1 (14.29) | −0.09 (−56.25) | ||
| V2-V0 | −0.03 (−7.5) | 0 | −0.53 (−88.33) | −0.2 (−28.57) | −0.09 (−56.25) | ||
| V3-V0 | 0.33 (82.5) | * | * | * | * | ||
| V4-V0 | −0.05 (−12.5) | 0.24 (60) | −0.44 (−73.33) | −0.44 (−62.86) | 0 | ||
| V5-V0 | 0 | 0.24 (60) | −0.44 (−73.33) | −0.44 (−62.86) | 0 | ||
| PPT | Anterior serratus (kg/m2) | V0 | 16 | 30.17 | 52 | 18.67 | 10.33 |
| V1-V0 | 4.1 (17.21) | 2.56 (6.29) | −21.33 (−41.02) | −5.34 (−27.63) | −0.21 (−0.10) | ||
| V2-V0 | 7.83 (32.86) | −3.67 (−9.02) | −28 (−53.85) | 0.66 (3.41) | 9.67 (48.35) | ||
| V3-V0 | −1.5 (−6.29) | * | * | * | * | ||
| V4-V0 | −3 (−12.59) | 10.5 (25.82) | −9.33 (−17.94) | −11.34 (−58.67) | 2 (10) | ||
| V5-V0 | −10.33 (−43.35) | −3.17 (−7.79) | −27 (−51.92) | −11.67 (−60.37) | −4.66 (−23.3) | ||
| Epicondyles (kg/m2) | V0 | 20.5 | 37.33 | 49.67 | 18.33 | 20 | |
| V1-V0 | 1.17 (4.94) | −7.66 (−18.68) | −15.34 (−30.88) | 4.34 (17.36) | −0.42 (−0.19) | ||
| V2-V0 | 3.17 (13.29) | −9.33 (−22.76) | −21.84 (−43.97) | 6.67 (26.68) | −2.17 (−10.01) | ||
| V3-V0 | −0.5 (−2.11) | * | * | * | * | ||
| V4-V0 | −7.5 (−31.9) | 3.67 (8.95) | −25 (−50.33) | 0.67 (2.68) | 1.67 (7.71) | ||
| V5-V0 | −15.17 (−64.09) | −4.5 (−10.98) | −23.34 (−46.99) | −1.66 (−6.64) | −13 (−59.99) | ||
| Vastus lateralis quadriceps (kg/m2) | V0 | 25.33 | 0 | 53.33 | 29.33 | 23.33 | |
| V1-V0 | 4 (13.04) | 64.67 (91.95) | −11.66 (−21.86) | 0.5 (1.27) | −23.33 (−71.41) | ||
| V2-V0 | 5.34 (17.41) | 44.83 (63.74) | −7.5 (−14.06) | 10 (25.43) | 9.34 (28.59) | ||
| V3-V0 | −5.33 (−17.38) | * | * | * | * | ||
| V4-V0 | −6.33 (−20.64) | 64.33 (91.47) | −19.33 (−36.25) | −6 (−15.26) | 7.67 (23.48) | ||
| V5-V0 | −5.66 (−18.45) | 70.33 (100) | −19.33 (−36.25) | −11.33 (−28.81) | −21 (−64.28) | ||
| Medium scalene (kg/m2) | V0 | 10 | 19.07 | 47.67 | 11.83 | 12.17 | |
| V1-V0 | 4 (28.57) | −3.74 (−19.61) | −32 (−67.13) | 1.17 (7.16) | −0.67 (−11.5) | ||
| V2-V0 | −3 (−21.43) | −3.07 (−16.1) | −27 (−56.64) | 4.5 (27.56) | 1.16 (8.7) | ||
| V3-V0 | −3.33 (−23.79) | * | * | * | * | ||
| V4-V0 | 0 | −5.74 (−30.1) | −19.34 (−40.57) | −1.83 (−11.21) | −1.5 (−11.25) | ||
| V5-V0 | −6 (−42.86) | −4.74 (−24.86) | −26.67 (−55.95) | −9.83 (−60.2) | −8.84 (−66.32) | ||
| Participants | ||||||
|---|---|---|---|---|---|---|
| Questionnaire | V | 1 | 2 | 3 | 4 | 5 |
| CSI (0–100) | V0 | 40 | 23 | 29 | 28 | 25 |
| V1-V0 | 9 (9) | −14 (−14) | −13 (−13) | 7 (7) | 4 (16) | |
| V2-V0 | * | −14 (−14) | −18 (−18) | −2 (−2) | 6 (6) | |
| V3-V0 | 2 (2) | * | * | * | * | |
| V4-V0 | 17 (17) | −5 (−5) | −19 (−19) | * | 20 (20) | |
| V5-V0 | 9 (9) | −14 (−14) | −14 (−14) | 5 (5) | 15 (15) | |
| LANSS (0–24) | V0 | 2 | NP | NP | 0 | NP |
| V1-V0 | 9 | 2 | 15 | 10 | 2 | |
| V2-V0 | * | 0 | 8 | 0 | 0 | |
| V3-V0 | 15 | * | * | * | * | |
| V4-V0 | 1 | 0 | 0 | * | 10 | |
| V5-V0 | 1 | 0 | 0 | 0 | 10 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo-Gallego, L.; Arranz-Martín, B.; Romay-Barrero, H.; Prieto-Gómez, V.; Lluch, E.; Torres-Lacomba, M. Changes in Pain Sensitivity in Treatment for Breast Cancer: A 12-Month Follow-Up Case Series. Int. J. Environ. Res. Public Health 2022, 19, 4055. https://doi.org/10.3390/ijerph19074055
Lorenzo-Gallego L, Arranz-Martín B, Romay-Barrero H, Prieto-Gómez V, Lluch E, Torres-Lacomba M. Changes in Pain Sensitivity in Treatment for Breast Cancer: A 12-Month Follow-Up Case Series. International Journal of Environmental Research and Public Health. 2022; 19(7):4055. https://doi.org/10.3390/ijerph19074055
Chicago/Turabian StyleLorenzo-Gallego, Laura, Beatriz Arranz-Martín, Helena Romay-Barrero, Virginia Prieto-Gómez, Enrique Lluch, and María Torres-Lacomba. 2022. "Changes in Pain Sensitivity in Treatment for Breast Cancer: A 12-Month Follow-Up Case Series" International Journal of Environmental Research and Public Health 19, no. 7: 4055. https://doi.org/10.3390/ijerph19074055
APA StyleLorenzo-Gallego, L., Arranz-Martín, B., Romay-Barrero, H., Prieto-Gómez, V., Lluch, E., & Torres-Lacomba, M. (2022). Changes in Pain Sensitivity in Treatment for Breast Cancer: A 12-Month Follow-Up Case Series. International Journal of Environmental Research and Public Health, 19(7), 4055. https://doi.org/10.3390/ijerph19074055








